Traditional therapies for traumatic brain injury are not enough. Could the historically vilified cannabis plant contain a solution?
Despite standard therapies, traumatic brain injuries (TBIs) contribute to about 30 percent of all injury deaths per year (1). In addition, it is common for TBI survivors to suffer long-term impairments in memory, hearing, vision, and emotional regulation. These factors place a heavy toll, not only on the person who suffered a TBI but also on their family.
However, research increasingly shows that cannabinoids may reduce the risk of developing a traumatic brain injury—and potentially reduce its devastating effects.
Yes, “cannabinoids” as in “cannabis.”
Weed
Reefer
Ganja
Mary Jane
Grass
Herb
You get the idea.

Cannabis Sativa
Photo credit By Thayne Tuason
Understanding Cannabinoids: The Basics
Cannabinoids are naturally occurring compounds found in the cannabis plant. At this point, you may be thinking that I fell off the hippie cannabis cruiser and got kidnapped by a group of twig munching, barefoot, crystal wearing, forest bathing hippies who tied me to their sacred tree and stuffed a lit joint in my mouth.
Not quite.
I fell down the cannabis hippie hole three years ago while investigating compounds that may reduce my personal risk of brain trauma. I spend a lot of my non-research rabbit-holing time in high-risk situations such as mountain biking, wakeboarding, and kiteboarding—I’m well aware that these activities carry the risk of head impact and traumatic brain injury.
When I first stumbled upon research pointing to the use of cannabinoids in preventing and treating TBI, I honestly did not pay much attention. I figured it was just “random hippie pot banter,” and I didn’t get past the abstracts.
It turns out I was wrong.
Really wrong.
Again and again, I kept seeing research suggesting that compounds found in cannabis could benefit TBI outcomes. And here’s the thing: You don’t have to smoke pot to get the benefits.
To really understand cannabinoids and their potential in addressing TBI prevalence and severity, let’s back up a bit and flesh out the science stuff. (Nerd-herder alert: We are going to get technical here dooooooode.)
TBI and the Brain’s Energy Crisis
Due to the nature of the insult, each TBI is unique. Pathological metabolic changes are common and can lead to further brain atrophy and cognitive decline (2).
In short, when you take a significant whack to your cabeza, your brain slams into your skull at high speed. The results of neurological gelatinous material hitting an incredibly dense bony cage can be crudely categorized into three groups depending on the type of damage produced:
1) Diffuse axonal injury (DAI) where the axons (think of them like complex computer wires) become torn/disrupted.
2) Focal contusions (aka, brain bruise).
3) Bleeding (hematoma) in/around the brain.
The other common but lesser-discussed fallout, in addition to the injury itself, is a massive disruption in glucose metabolism. Within seconds after an impact, your brain’s primary source of energy, glucose, is FUBAR’d, and a whole host of metabolic and enzymatic changes spring into action (3, 4). As a result, your brain paradoxically experiences a short-lived increase in glucose uptake, followed by a much longer period of glucose depression (5).
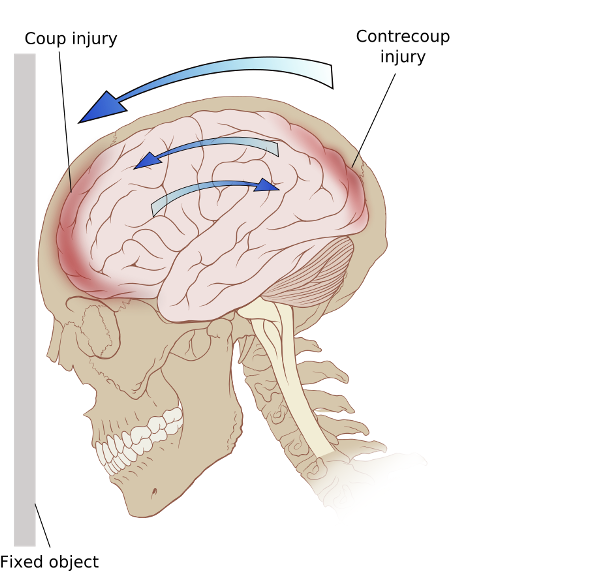
Ricochet of the brain within the skull may account for the coup-contrecoup phenomenon
Photo credit: Modified version of Image:Skull and brain normal human.svg by Patrick J. Lynch, medical illustrator
The brain is unique in that it uses glucose as its primary fuel source—it cannot use fat directly for fuel. Similar to muscle tissue, your brain stores this glucose in the form of glycogen. However, the amount of stored glucose as glycogen in the brain is minimal (6). When brain glycogen levels become depleted (such as in TBI), the main fuel for the brain quickly gets exhausted, thus manifesting in an extreme energy crisis.
Imagine driving a diesel car in a foreign country that does not have diesel fuel at the gas stations. Once you run out of fuel in your tank, you are hosed. While the brain can technically switch over to a different energy source called ketones, they are typically in short supply at the time of injury.
Ketones are derived from fatty acid metabolism, mainly occurring in the liver. The production of ketones can be increased via the ketogenic diet or periods of long-term fasting (7). The body produces three different types of ketones—3-β-hydroxybutyrate (3BHM/BHB), acetoacetate, and acetone, (8) known collectively as “ketone bodies” (5, 9, 10). The downside is that the production of ketones takes several hours to days to potentially weeks in some people to become high enough to serve as an alternative fuel for the damaged brain. In addition, the ketogenic diet is very, very low in carbohydrates (less than a bagel per day), making compliance hard.
Is there another compound that could be administered post-TBI or consumed as part of the daily diet to help mitigate TBI issues in a much shorter timeframe?
How Cannabinoids Support TBI Recovery
Before we discuss how cannabinoids may support TBIs, it’s essential to realize that, so far, researchers have isolated and identified more than 100 cannabinoids in cannabis.
Cannabinoids are classified as:
- Phytocannabinoids – Cannabinoids found in leaves, flowers, stems, and seeds collected from the Cannabis sativa plant.
- Endogenous – Cannabinoids made by the body—examples include N-arachidonoylethanolamine or anandamide (AE) or 2-arachidonoylglycerol (2-AG). AE and 2-AG activity can be manipulated by inhibiting their corresponding hydrolases FAAH or MAGL, preventing their degradation.
- Purified – Compounds isolated from plant sources—examples include cannabidiol (CBD) and delta-9-tetrahydrocannabinol (THC).
- Synthetic – Cannabinoids synthesized in a laboratory—examples include CB1 agonists (CPP-55, ACPA), CB2 agonists (JWH-133, NMP7, AM1241), CB1/CB2 nonselective agonist (CP55940), ajulemic acid (AJA), nabilone, and dronabinol (11)
Under the “endogenous” category, you may have noticed that your body makes cannabinoids! Shhh, don’t tell anyone you are carrying them. The accumulating data demonstrates that these compounds appear to be beneficial for multiple physiologic systems due to the pervasive nature of the endocannabinoid system.
Cannabis sativa, scientific drawing from c1900
Photo credit: Walther Otto Müller - From Franz Eugen Köhler's Medizinal-Pflantzen. Published and copyrighted by Gera-Untermhaus, FE Köhler in 1887 (1883–1914).
In 2005, Mechoulam, R. (12) suggested that cannabinoids from plants could interact with the endogenous system. He cites that anandamide and 2-arachidonoyl glycerol may play a role not only in the central nervous system but also in most physiological systems that have been investigated—the immune, cardiovascular, reproductive, respiratory, and skeletal systems. Additionally, they may influence insulin sensitivity, fat and energy metabolism, metabolic syndrome, and augment the benefits of physical exercise (13, 14).
Out of the hundreds of cannabinoids, only one has psychoactive properties—tetrahydrocannabinol, aka THC. This is the compound that makes you feel spacey/stoned.
While it is rumored that the Chinese used cannabis since the first century AD (15), THC was first isolated by Mechoulam and colleagues in 1964. This discovery allowed researchers to begin untangling the complex endocannabinoid system (16, 17). In 2015, Baron, E.P. (18) stated that the cannabis plant contains a vast number of pharmacological and biochemical compounds, of which only a minority are understood.
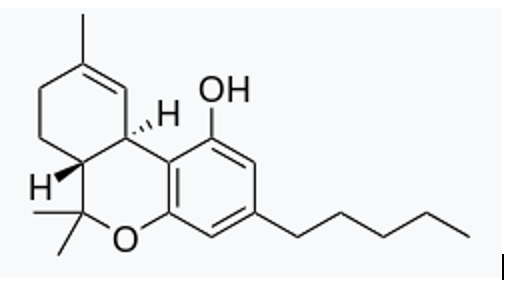
THC Structure
By Harbin - Own work, Public Domain
Metabolic Energy Crisis and Blood Brain Barrier
When investigating any new compound for its effects on the brain, the first task that it must pass the muster on is to ensure that it will cross the blood-brain barrier (BBB). As anyone who has used THC can tell you, it does. THC does pass through the BBB, as do most of the other cannabinoids.
We know that closed head injuries cause major transient disruption of the BBB integrity, which peaks four hours post-injury (11). When TBI occurs, your BBB opens as wide as your mouth when the dentist is going to cap a tooth. This “leaky” BBB allows everything to flow into your brain, including all the nasty things you do not want there. The brain can then run amok with massive amounts of inflammation(11). Amazingly, cannabinoids may serve to help protect the blood-brain barrier itself, thus reducing inflammation while serving up antioxidant properties, too (19, 20).
Chen et al. 2000 state, “…the antioxidative property of cannabinoids was confirmed by their ability to antagonize oxidative stress and consequent cell death induced by the retinoid anhydroretinol. Therefore, cannabinoids act as antioxidants to modulate cell survival and growth of B lymphocytes and fibroblasts (21).”
Imagine that your home was broken into by a bunch of thieves looking for money. Being the super-smart homo sapien you are, you hid your money in a secret compartment at the back of your closet. The thieves can’t find the money and start trashing your home. First, your home gets broken into. Second, your home gets trashed.
Even worse is that your home now has damage and the thieves found your money! This analogy is what happens post-TBI—your BBB opens up due to injury, your brain is inundated with noxious inflammatory compounds that trash the place, and the energy supply is disrupted.
While the data is sparse and preliminary, it is quite promising for the use of cannabinoids in the reduction of risk from TBI, as they serve two purposes: They act as both a better deadbolt on the door (BBB integrity) and as the cleaning crew (mopping up neuroinflammation), all in one.
Mechanism of Cannabinoids
Endogenous cannabinoids produce their effects by acting on two different receptor types throughout the body: Type-1 cannabinoid receptor (CB1) and type-2 cannabinoid receptor (CB2). Current research (22) indicates that endocannabinoids are involved in many different processes, including neuronal survival after ischemia or trauma.
In 2014, researchers provided anatomical and biochemical evidence that functional CB1 receptors are located within brain mitochondria, suggesting an inferred and direct relationship between the CB1 receptor and mitochondrial functions in the brain (23). CB1 receptors are also found on neuronal mitochondrial outer membranes (mtCB1), which influence the aerobic respiration pathway in the creation of cellular energy (ATP).
Work from Xu et al. (24) in TBI-induced fuzzy mice found that mtCB1 quickly upregulated after TBI. An ATP shortage accompanied this upregulation via a reduction of mitochondrial respiration of about 20–30% (23, 25, 26). The ATP shortage paradoxically protected neurons from apoptosis (cell death). When mtCB1 was activated, it upregulated mitochondrial AKT/complex V activity to help reverse the ATP shortage.
Argh….English, you nerd—do you speak it?!
When you translate the geek-speak, this means that mtCB1 is influenced by TBI and is a potential molecular target for cannabinoids.
A Whack To The Head
Upon getting cracked on the head, full-scale changes appear to include cannabinoid receptors (CBRs). In a study done on 13 piglets (27) subjected to a sham operation or fluid percussion (FP) injury, researchers found evidence of binding changes of CB2R density. However, CB1R density was not affected (27). It may be that different types of TBI affect different endogenous cannabinoid receptors differently. And, since THC is known to interact with the CB1 receptor, could it be a valid therapeutic mechanism?
Maybe.
THC (and anandamide) may act as efficacious CB1 receptor agonists in neuronal tissue (29). But, the science gets complex and messy fast, so hang on.
Lu H.C. et al. (2016) stated, “…the interactions of THC, CB1 and the endocannabinoids are more complex than THC simply ‘hijacking’ CB1 receptors as another agonist and need to be carefully considered (28).”
However, the administration of a CB1R agonist (ACEA—arachidonyl-2″-chloroethylamide, to be specific) after a moderately severe experimental TBI rescued both learning and memory abilities in young adult male rats (30). This data provides evidence that acting on the CB1 receptor may help improve brain functions post-TBI. Yes, this was a rat study—humans volunteering for head-whacks are in short supply (31).
Further down the receptor pathway, does THC specifically affect the mtCB1 receptor? If so, this may be an intervention for those with TBI.
To date, we don’t have any direct data on THC or cannabinoids on mtCB-1 receptors in the brain, but there was a study looking at their interactions in cardiac muscle tissue (26). The researchers found that the mtCB1 receptor was active and application of a THC treatment decreased mitochondria coupled respiration between 12 and 15 percent in wild-type isolated myocardial mitochondria (26). In summary, it appears that THC does have a significant effect on cardiac tissue, and future work would need to be conducted on brain tissue to confirm any potential impact.
Human THC and TBI Data
Since THC remains illegal at the Federal level at the time of this writing, performing a randomized prospective trial would be very difficult. However, retrospective investigations are possible. In one such study, a three-year retrospective review was performed on data from 446 patients who sustained a TBI (32). The researchers determined how many TBI sufferers also tested positive for THC. They compared the THC-positive group with the THC-negative group for injury mechanism, severity, disposition, and mortality. They concluded that a positive THC screen was associated with a decreased mortality in adult patients sustaining TBI (32).
Before your next bong rip, keep in mind that the limitations of this study are many. The exact dosage and time course were impossible to state. THC is also fat-soluble, and while most will be excreted within less than a week, a small amount will be present for an extended time. Despite these limitations, this study does provide some human data that THC is associated with better TBI outcomes (32).
There is also evidence from one synthetic cannabinoid studied in humans for TBI called dexanabinol, aka HU211. While the initial animal models produced great data (33), HU211 ultimately failed to show any long-term benefit. Interestingly, while dexanabinol is technically an enantiomer of the potent synthetic cannabinoid agonist HU210, it paradoxically does not bind or activate cannabinoid receptors (31).
Other cannabinoid compounds such as 2-AG may partially exert neuroprotection by inhibiting the early (1 to 4 hours) inflammatory response and augmentation of (the brain reducing power) (clarify, please) (34). In addition, cannabis contains hundreds of other cannabinoids beyond just one sub-type. Thus, while interesting, this data adds to the complexity of cannabinoid therapy and opens up a future area ripe for more research.
Are Cannabinoids Safe?
It appears cannabinoids have some beneficial properties, especially when taken before a bonk on the head—but are they safe? While all drugs have side effects, cannabinoids have a favorable safety profile, with the only psychoactive compounds being THC (35-40).
Turner A.R. et al. stated, “For obvious ethical reasons, there is no experimental evidence to determine the lethal dose in humans; but the dose that kills animals ranges from 40 mg/kg to 130 mg/kg intravenously (41).”
Applying the above animal dosage directly to a 100 kg (220 lb) human would be an acute dose via an IV supplying 400 mg on the low end. Oral THC is only 4% to 12% bioavailable, and absorption is highly variable in edible cannabis products (42). If we take the simpleton math of 10%, you would need an ingested dose of around 4,000 mg. That is beyond a heroic amount.
Keep in mind that the minimal labeled dose in many states is either 5 or 10 mg on edible products. Even if you ingested a product with a massive amount of THC—in the 200 mg range, for example, enough to send most people to the moon and beyond—you would still need to ingest about 20 of them to get close to the LD50. This is not recommended, of course. In short, while you may feel like you are dying and time has stopped, the therapeutic window of cannabinoids is enormous, and your risk of actual death is very low.
Cannabinoid Benefits Without the THC?
So far, it appears cannabinoids, and THC, specifically, could benefit TBIs through multiple mechanisms.
What do you do if your state has declared marijuana (cannabis) illegal?
Hemp Oil
Hemp oil is just as the name sounds—oil made from the hemp plant. Hemp is actually a cannabis plant with low concentrations of THC. Over time, breeders of cannabis have sought higher and higher amounts of THC. The cannabis strains that were super low in THC used to be called “the hippie’s disappointment” since you could smoke it all day and never get stoned due to the low THC content.
Relatively recently, hemp was classified under the Department of Agriculture and can be grown as a crop. Previously, it was in a legal gray area as THC in cannabis is still federally illegal. Hemp must contain less than 0.3% THC content by dry weight. Despite the very low THC content, it can still contain many other beneficial compounds and is currently legal in all 50 states. Therefore, you can get the benefits of these phytonutrients even if your state has not legalized marijuana. This option is also great as many don’t want to get stoned taking a supplement.
My First-Hand Cannabis Field Trip
I wanted to get more information on how hemp oil and cannabinoids are manufactured. So, a few years ago, I ventured out to Colorado to check out the company Charlotte’s Web as they manufacture hemp oil as a supplement. I was able to secure a tour of their facility, which was amazing and top-notch all the way. In fairness, I had to sign a non-disclosure agreement, so I can’t tell you everything about it. However, what impressed me the most was their dedication to quality at every step in the process.
At Charlotte’s Web, they start with a clone (not seed) of the hemp strain they use (fittingly called Charlotte’s Web). The benefit of hacking off part of a plant, getting it to sprout, and then planting it by hand is that the exact same strain (genetic material) is always used. All the plants are weeded and harvested by hand using organic farming methods and grown on specific farms to ensure oversight in every step of the process.

My buddy Jason Rule of Driven Nutrition describes it as steak versus a hamburger. A steak is from only one cow, but a hamburger could be from many cows. Many hemp oil /CBD manufacturers take different hemp strains, mix them all together, and then sort it out on the back end (assuming they standardize for specific compounds like CBD). Charlotte’s Web is more like a steak—all the plant material is of identical make-up, thus ensuring a higher quality product.
The hemp is harvested and then turned into oil via a process called decarboxylation. Even if you were to wander through a marijuana field in Colorado, munching on the plant leaves like some rabbit-journeyman, your body’s THC blood concentrations would not rise.
This is because THC (and other cannabis compounds) need to undergo a chemical process (decarboxylation) to transform them into beneficial compounds. Marijuana is heated in different ways (lighter, vaporizer, etc.) for decarboxylation to take place and for the right compounds to form.
The two main methods of decarb (industry term) are heat and time, although alcohol and CO2 extraction can also be used. Very minimal decarb occurs from drying and curing. You can also get fancy and use pressure to change the time and heating points if you remember your college physics.
“Decarboxylation is an important step for efficient production of the major active components in cannabis, for example, Δ9-tetrahydrocannabinol (Δ9-THC), cannabidiol (CBD), and cannabigerol (CBG). These cannabinoids do not occur in significant concentrations in cannabis but can be formed by decarboxylation of their corresponding acids, the predominant cannabinoids in the plant.”
– Wang, M.,et al. 2016 (43)
Once decarboxylation is complete, therapeutic compounds are concentrated to the correct amount and the hemp oil is bottled. Along the way, there are multiple quality controls, or QCs, procedures to help ensure a super-pure product.
The benefit? You get polyphenol-rich hemp oil, containing less than 0.3 percent THC, and it can be shipped to all 50 states.
Summary
The research on cannabinoids’ role in TBI support is in its infancy. But, there is some good data that the reward/risk ratio is quite positive. If you live in a location where cannabis is legal, compounds that have a bit higher THC are a realistic option. If that is not an option, the use of high-quality hemp oil (standardized for CBD) is another alternative.
As always, talk to your doctor or functional neurologist about what is best for you and your specific condition.
In retrospect, it’s astounding that I managed to overlook an entire class of beneficial and researched plant phytonutrients, with evidence on how they affect almost every system in the body. Unfortunately, I let my preconceived notions prevent me from doing my own research.
While the direct data on the effect of cannabinoids on TBI both before and after the injury is still preliminary, the safety profile and potential for benefit are massive.
Sometimes, the hippies are right.
References
1. Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths – United States, 2007 and 2013. Morbidity and mortality weekly report Surveillance summaries (Washington, DC : 2002). 2017;66(9):1-16.
2. Wright MJ, McArthur DL, Alger JR, Van Horn J, Irimia A, Filippou M, et al. Early metabolic crisis-related brain atrophy and cognition in traumatic brain injury. Brain imaging and behavior. 2013;7(3):307-15.
3. Vespa P, Bergsneider M, Hattori N, Wu HM, Huang SC, Martin NA, et al. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2005;25(6):763-74.
4. Glenn TC, Kelly DF, Boscardin WJ, McArthur DL, Vespa P, Oertel M, et al. Energy dysfunction as a predictor of outcome after moderate or severe head injury: indices of oxygen, glucose, and lactate metabolism. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2003;23(10):1239-50.
5. Prins ML, Matsumoto JH. The collective therapeutic potential of cerebral ketone metabolism in traumatic brain injury. Journal of lipid research. 2014;55(12):2450-7.
6. Falkowska A, Gutowska I, Goschorska M, Nowacki P, Chlubek D, Baranowska-Bosiacka I. Energy Metabolism of the Brain, Including the Cooperation between Astrocytes and Neurons, Especially in the Context of Glycogen Metabolism. International journal of molecular sciences. 2015;16(11):25959-81.
7. Paoli A, Bianco A, Damiani E, Bosco G. Ketogenic diet in neuromuscular and neurodegenerative diseases. BioMed research international. 2014;2014:474296.
8. Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes/metabolism research and reviews. 1999;15(6):412-26.
9. Prins ML, Matsumoto J. Metabolic Response of Pediatric Traumatic Brain Injury. Journal of child neurology. 2016;31(1):28-34.
10. Orhan N, Ugur Yilmaz C, Ekizoglu O, Ahishali B, Kucuk M, Arican N, et al. Effects of beta-hydroxybutyrate on brain vascular permeability in rats with traumatic brain injury. Brain research. 2016;1631:113-26.
11. Chen Y, Constantini S, Trembovler V, Weinstock M, Shohami E. An experimental model of closed head injury in mice: pathophysiology, histopathology, and cognitive deficits. Journal of neurotrauma. 1996;13(10):557-68.
12. Mechoulam R. Plant cannabinoids: a neglected pharmacological treasure trove. Br J Pharmacol. 2005;146(7):913-5.
13. Tantimonaco M, Ceci R, Sabatini S, Catani MV, Rossi A, Gasperi V, et al. Physical activity and the endocannabinoid system: an overview. Cellular and molecular life sciences : CMLS. 2014;71(14):2681-98.
14. Pagotto U, Pasquali R. Endocannabinoids and energy metabolism. Journal of endocrinological investigation. 2006;29(3 Suppl):66-76.
15. Brand EJ, Zhao Z. Cannabis in Chinese Medicine: Are Some Traditional Indications Referenced in Ancient Literature Related to Cannabinoids? Front Pharmacol. 2017;8:108.
16. Tasker JG, Chen C, Fisher MO, Fu X, Rainville JR, Weiss GL. Endocannabinoid Regulation of Neuroendocrine Systems. Int Rev Neurobiol. 2015;125:163-201.
17. Gaoni Y, Mechoulam R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. Journal of the American Chemical Society. 1964;86(8):1646-7.
18. Baron EP. Comprehensive Review of Medicinal Marijuana, Cannabinoids, and Therapeutic Implications in Medicine and Headache: What a Long Strange Trip It’s Been. Headache. 2015;55(6):885-916.
19. Vendel E, de Lange EC. Functions of the CB1 and CB 2 receptors in neuroprotection at the level of the blood-brain barrier. Neuromolecular medicine. 2014;16(3):620-42.
20. Ranieri R, Laezza C, Bifulco M, Marasco D, Malfitano AM. Cannabinoids and Neuro-Inflammation: Regulation of Brain Immune Response. Recent patents on CNS drug discovery. 2016;10(2):178-203.
21. Chen Y, Buck J. Cannabinoids protect cells from oxidative cell death: a receptor-independent mechanism. The Journal of pharmacology and experimental therapeutics. 2000;293(3):807-12.
22. Panikashvili D, Simeonidou C, Ben-Shabat S, Hanus L, Breuer A, Mechoulam R, et al. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413(6855):527-31.
23. Hebert-Chatelain E, Reguero L, Puente N, Lutz B, Chaouloff F, Rossignol R, et al. Cannabinoid control of brain bioenergetics: Exploring the subcellular localization of the CB1 receptor. Molecular metabolism. 2014;3(4):495-504.
24. Xu Z, Lv XA, Dai Q, Ge YQ, Xu J. Acute upregulation of neuronal mitochondrial type-1 cannabinoid receptor and it’s role in metabolic defects and neuronal apoptosis after TBI. Molecular brain. 2016;9(1):75.
25. Benard G, Massa F, Puente N, Lourenco J, Bellocchio L, Soria-Gomez E, et al. Mitochondrial CB(1) receptors regulate neuronal energy metabolism. Nature neuroscience. 2012;15(4):558-64.
26. Mendizabal-Zubiaga J, Melser S, Benard G, Ramos A, Reguero L, Arrabal S, et al. Cannabinoid CB1 Receptors Are Localized in Striated Muscle Mitochondria and Regulate Mitochondrial Respiration. Front Physiol. 2016;7:476.
27. Donat CK, Fischer F, Walter B, Deuther-Conrad W, Brodhun M, Bauer R, et al. Early increase of cannabinoid receptor density after experimental traumatic brain injury in the newborn piglet. Acta Neurobiol Exp (Wars). 2014;74(2):197-210.
28. Lu HC, Mackie K. An Introduction to the Endogenous Cannabinoid System. Biological psychiatry. 2016;79(7):516-25.
29. Laaris N, Good CH, Lupica CR. Delta9-tetrahydrocannabinol is a full agonist at CB1 receptors on GABA neuron axon terminals in the hippocampus. Neuropharmacology. 2010;59(1-2):121-7.
30. Arain M, Khan M, Craig L, Nakanishi ST. Cannabinoid agonist rescues learning and memory after a traumatic brain injury. Ann Clin Transl Neurol. 2015;2(3):289-94.
31. Schurman LD, Lichtman AH. Endocannabinoids: A Promising Impact for Traumatic Brain Injury. Front Pharmacol. 2017;8:69.
32. Nguyen BM, Kim D, Bricker S, Bongard F, Neville A, Putnam B, et al. Effect of marijuana use on outcomes in traumatic brain injury. The American surgeon. 2014;80(10):979-83.
33. Nadler V, Biegon A, Beit-Yannai E, Adamchik J, Shohami E. 45Ca accumulation in rat brain after closed head injury; attenuation by the novel neuroprotective agent HU-211. Brain research. 1995;685(1-2):1-11.
34. Panikashvili D, Shein NA, Mechoulam R, Trembovler V, Kohen R, Alexandrovich A, et al. The endocannabinoid 2-AG protects the blood-brain barrier after closed head injury and inhibits mRNA expression of proinflammatory cytokines. Neurobiol Dis. 2006;22(2):257-64.
35. Ahmed AI, van den Elsen GA, Colbers A, van der Marck MA, Burger DM, Feuth TB, et al. Safety and pharmacokinetics of oral delta-9-tetrahydrocannabinol in healthy older subjects: a randomized controlled trial. Eur Neuropsychopharmacol. 2014;24(9):1475-82.
36. van den Elsen GA, Ahmed AI, Lammers M, Kramers C, Verkes RJ, van der Marck MA, et al. Efficacy and safety of medical cannabinoids in older subjects: a systematic review. Ageing research reviews. 2014;14:56-64.
37. Sachs J, McGlade E, Yurgelun-Todd D. Safety and Toxicology of Cannabinoids. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2015;12(4):735-46.
38. Kersten BP, McLaughlin ME. Toxicology and management of novel psychoactive drugs. Journal of pharmacy practice. 2015;28(1):50-65.
39. Sarne Y, Mechoulam R. Cannabinoids: between neuroprotection and neurotoxicity. Current drug targets CNS and neurological disorders. 2005;4(6):677-84.
40. Forney RB. Toxicology of marihuana. Pharmacol Rev. 1971;23(4):279-84.
41. Turner AR AS. Marijuna Toxicity. StatPearls, editor. FL: Treasure Island; Updated 2018 Oct 27.
42. McGilveray IJ. Pharmacokinetics of cannabinoids. Pain research & management. 2005;10 Suppl A:15a-22a.
43. Wang M, Wang YH, Avula B, Radwan MM, Wanas AS, van Antwerp J, et al. Decarboxylation Study of Acidic Cannabinoids: A Novel Approach Using Ultra-High-Performance Supercritical Fluid Chromatography/Photodiode Array-Mass Spectrometry. Cannabis Cannabinoid Res. 2016;1(1):262-71.





Leave A Comment